Naturstoffe aus Cyanobakterien
Cyanobakterien sind eine wenig untersuchte Quelle von chemisch hoch interessanten Naturstoffen mit vielfältigen und potenten biologischen Aktivitäten.
Wir haben eine Reihe von Projekten, in denen wir uns mit cyanobakteriellen Naturstoffen beschäftigen.
Microcystine als Leitstrukturen
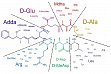
Microcystine, zyklische Heptapeptide aus Cyanobakterien, können möglicherweise als Leitstrukturen für die Entwicklung von Wirkstoffen dienen, mit denen einige Krebsarten gezielt bekämpft werden können. Zusammen mit der Firma Cyano Biotech GmbH (Berlin) arbeiten wir an der Optimierung der Microcystin-Grundstruktur und der Entwicklung von Antikörper-Wirkstoff-Konjugaten mit dem Ziel einer selektiven Wirkung der Substanzen gegen Krebszellen, so dass ihre allgemeine Toxizität abgeschwächt und damit die Nebenwirkungen vermindert werden
Microcystine haben einen in der Krebstherapie neuen Wirkmechanismus (Hemmung von Proteinphosphatasen) und können erst nach aktivem Transport in Zellen toxisch wirken. Wir haben die chemische Struktur der Microcystine so modizifiziert, dass sie an Antikörper gebunden werden können, die nur von Krebszellen aufgenommen werden können.
Diese neuen Microcystine können auch leicht mit jeder Art von Markern verknüpft werden und dann z.B. Elektronen- oder Fluoreszenzmikroskopisch untersucht werden.
Diese Substanzen wollen wir als biochemische Werkzeuge nutzen, um in verschiedenen Studien die Rolle der Microcystine für die produizierenden Organismen aufzuklären. Mit Hilfe dieser Verbindungen können auch die Wirkung der Microcystine auf eukaryotische Zellen und ihre zelluläre Aufnahme untersucht werden.
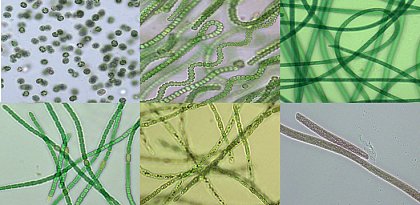
Microcystin-produzierende Cyanobakterien-Stämme

Das Projekt wurde vom BMWi finanziell unterstützt (2017-2019) und von Dr. Julia Moschny bearbeitet

Aktuell arbeiten Laura Stock und Lena Höper (gefördert von der DFG) an diesem Projekt.
Kooperationen:
- Dr. Dan Enke, Cyano Biotech GmbH, Berlin
- Prof. Frank Bordusa, Naturstoffbiochemie, Uni Halle-Wittenberg
Charakterisierung des "Adlermörder-Gifts" Aetokthonotoxin
Die Avian Vacuolar Myelinopathy (AVM) ist eine tödlich verlaufende neurologische Erkrankung, die hauptsächlich Vögel (und einige Reptilien) befällt und die in den letzten Jahren verstärkt in den südöstlichen Bundesstaaten der USA auftritt. Typisch für diese Erkrankung sind Läsionen in verschiedenen Gehirnregionen betroffener Tiere. Ihr berühmtestes Opfer ist das nationale Symbol der Vereinigten Staaten, der Weißkopfseeadler. Die Ätiologie dieser Erkrankung war lange Zeit ungeklärt, aber kürzlich konnte von unseren amerikanischen Kooperationspartnern die Hypothese aufgestellt werden, dass über die Nahrungskette aufgenommene Cyanobakterien der neu beschriebenen Art Aetokthonos hydrillicola ursächlich für die neurologischen Schäden sind. Aetokthonos wächst epiphytisch auf der invasiven Wasserpflanze Hydrilla verticillata, welche sich aggressiv in den südlichen US-Bundesstaaten verbreitet. Dies legte die Vermutung nahe, dass AVM von einem cyanobakteriellen Toxin verursacht wird. Wir konnten das Cyanobakterium isolieren und in Kultur bringen und das für die AVM verantwortliche Toxin identifizieren. Aetokthonotoxin ist ein chemisch neuartiges Cyanotoxin, das im März 2021 als Titelgeschichte in der Zeitschrift Science veröffentlicht wurde.
Inzwischen steht auch eine Totalsynthese des Toxins zur Verfügung.
Aktuell bearbeiten wir die folgenden Fragen:
- Wie ist der Wirkmechanismus des Toxins?
- Welche Strukturmerkmale sind wichtig für die biologische AKtivität?
- Welche Umweltbedingungen haben Einfluss auf die Biosynthese?
- Wie wird das Toxin biosynthetisiert?
- Welche anderen Substanzen werden von dem Cyanobakterium Aetokthonos hydrillicola produziert?

Dieses Projekt wird von der DFG gefördert (2019-2022) und von Steffen Breinlinger und Markus Schwark bearbeitet.
Kooperationen:
- Prof. Susan Wilde, Warnell School of Forestry and Natural Resources, University of Georgia, Athens, USA
- Prof. James Lauderdale, Department of Genetics, University of Georgia, Athens, USA
- Jan Mareš, The Czech Academy of Sciences, Institute of Hydrobiology, České Budějovice, Czech Republic
- Pavel Hrouzek, The Czech Academy of Sciences, Center Algatech, Institute of Microbiology,Třeboň, Czech Republic
Halogenierte Naturstoffe aus Cyanobakterien
Cyanobakterien produzieren im Vergleich mit anderen Organismengruppen eine Vielzahl halogenierter Naturstoffe, die sich häufig durch ausgeprägte biologische Aktivität auszeichnen.
In diesem Projekt beschäftigen wir uns mit der gezielten Isolierung, Strukturaufklärung und biologischen Testung neuer halogenierter Naturstoffe und Charakterisieren ihre Produktion und Biosynthese.
Dieses Projekt wird von Franziska Schanbacher bearbeitet.
Abgeschlossene Projekte
ANoBIn - Antiinfektiva aus indonesischen Cyanobakterien

Indonesien ist ein sogannter "Biodiversitäts-Hotspot" mit einer dichten und artenreichen Flora und Fauna. In dem Deutsch-Indonesischen Kooperationsprojekt ANoBIn (Accessing Novel Bacterial Producers from Biodiversity-rich Habitats in Indonesia) suchten wir nach neuen antiinfektiven Naturstoffen, die von Cyanobakterien aus indonesischen Habitaten biosynthetisiert werden. Nach einer Probenahme-Exkursion und der Isolierung von Cyanobakterienstämmen aus den gesammelten Proben wurden diese Stämme identifiziert und taxonomisch eingeordnet sowie auf die Produktion von Naturstoffen untersucht. Anschließend wurden die interessantesten Stämme im größeren Maßstab kultiviert, extrahiert, und die Naturstoffe isoliert und ihre chemische Struktur aufgeklärt. Dieses Projekt wurde finanziell unterstützt vom Bundesministerium für Bildung und Forschung (BMBF).
Dabei suchten wir nicht nur nach neuen Naturstoffen mit direkten antibakteriellen oder antimykotischen Aktivitäten, sondern interessierten uns auch für Hemmstoffe von Proteasen, die aktuell bei bestimmten Infektionserregern als mögliche Angriffspunkte für neuartige Wirkstoffe diskutiert werden. So suchten wir z.B. nach Hemmstoffen der Protease Rhodesain von Trypanosoma brucei, dem Erreger der Schlafkrankheit.

Das Projekt wurde vom BMBF finanziell unterstützt und von Dr. Ronja Kossack bearbeitet.
Kooperationen:
- Prof. Dr. Jörg Overmann; Leibniz-Institut DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen)
- Prof. Dr. Peter Kämpfer; Institut für Angewandte Mikrobiologie, Justus-Liebig-Universität Giessen
- Prof. Dr. Stefan Schulz; Institut für Organische Chemie, Technische Universität Braunschweig
- Dr. Wien Kusharyoto; Laboratory for Applied Genetic Engineering and Protein Design, Indonesian Institute of Sciences (LIPI)
- Dr. Dwi Dwi Susilaningsih Setyawan; Research Center for Biotechnology, Indonesian Institute of Sciences (LIPI)
- Prof. Dr. Tanja Schirmeister, Institut für Pharmazie und Biochemie, Johannes Gutenberg Universität Mainz
Antibakterielle Cyclophane aus Nostoc

In Kooperation mit Wissenschaftlern der Universität Greifswald (M. Preisitsch und S. Mundt) haben wir an der Strukturaufklärung und Charakterisierung von Cyclophanen aus Nostoc gearbeitet. Ein Screening von Extrakten von Nostoc sp. zeigte, dass einige der Stämme aktiv gegen grampositive krankheitserregende Mikroorganismen wie S. aureus waren. Die aktiven Verbindungen wurden isoliert und ihre Struktur aufgeklärt - es handelte sich um 5 neue und 11 bereits bekannte Cyclophanderivate . Zusätzlich zu ihrer ausgeprägten antibakteriellen Aktivität gegen S. aureus und S. pneumoniae erwiesen sich die Verbindungen als zytotoxisch. Die Anwesenheit einer Carbamoyl-Gruppe verstärkte beide Aktivitäten leicht.
Wir haben Derivate der Naturstoffe produziert und vergleichende Bioaktivitätstests durchgeführt. Weiterhin haben wir in einem Screening von mehr als 100 Cyanobakterienstämmen nach neuen Cyclophanen gesucht. Dieses Screening führte zu der Entdeckung der Cylindrofridine , neuer Verbindungen mit interessanter Struktur, die uns dabei half, zur Aufklärung des Biosynthesewegs der Cyclophane beizutragen.

Dieses Projekt wurde finanziell vom DZIF unterstützt.




